
Learning Community > Understanding Aquatic Ecosystems > 1. Basics of Freshwater Ecosystems
Welcome to the fascinating and wonderful world of aquariums and fish keeping! Here, at That Aquarium we aspire to embark on this journey together with you via this virtual guide; offering both guidelines for the new hobbyists, as well as juicy tidbits of information for the more advanced aquarists. We hope that you will have fun navigating our website and be enthralled by the charm of the aquarium hobby which has captivated many from all walks of life. So, come with us, as we help to guide you towards success with our team of expert aquarists who are just a click away, always by your side!

“To Know Mother Nature is to love her smallest creations’’. -Takashi Amano No true words have been spoken other than those of the Mr. Takashi Amano, Nature photographer and founder of the Nature Aquarium style of planted aquariums which took the world by storm. Mother nature is both mysterious and magnanimous. And in our setting up an aquarium, inevitably we are re-creating a small slice of Nature within a glass box. Along with the various natural processes vital for sustaining life, as Nature intended. Thus, let us explore Mother Nature and learn from Her. Beginning with the element that brings life-water.
Water. Scientifically classed as the compound known as H2O and also dubbed as the “element” that brings life “across cultures, water exists in Nature as part of the water cycle. Where it is continuously purified and made available for sustaining organic life; both terrestrial and aquatic. A staggering 97% of the Earth’s water makes up the ocean, where it covers more than 70% of our planet’s surface. With the remaining 3% of that water being freshwater. The latter is characterised by lakes, rivers and streams, among others and it is in these types of freshwater habitats do our beloved tropical aquarium fishes abound, as well as their less well-represented coldwater congeners (e.g. sticklebacks and bitterings).

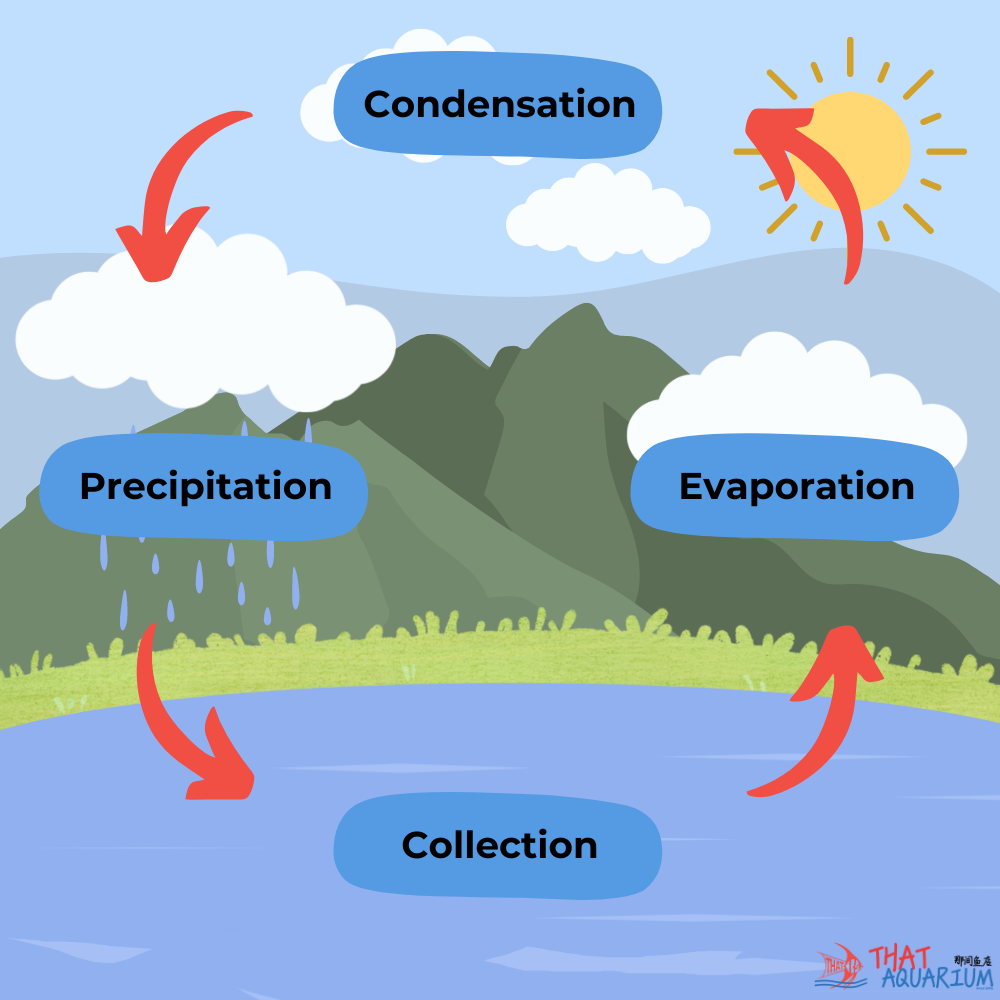
By understanding the water cycle, as it happens in Nature we will also learn of how each process alters the physical state and chemical composition of water. For it is the final end product that ends up in our aquariums and thus, the water chemistry of various municipal water supplies may be ideal for certain species of aquariums fishes but not for others.
Firstly, water evaporates into the air from open bodies of water i.e. ‘collection points’. Water in its gaseous state is pure H2O and thus free from dissolved compounds. As it rises into the atmosphere to form clouds, however the water will inevitably become slightly acidic. This is due to the presence of Co2 in the air which naturally dissolves into the water to form carbonic acid. This natural process is not to be confused with acid rain brought upon by the dissolution of toxic air pollutants such as sulphur dioxide and nitrogen oxides; which turns the water excessively acidic that can harm both terrestrial and aquatic life. It is here that we derive the first measurable water parameter, pH. Which indicates how acidic (< pH 7.0) or alkaline (> pH 7.0) water can be.
Science Time: Water pH stands for “potential of Hydrogen” or “power of Hydrogen.” It is a measure of the hydrogen ion concentration in water, indicating its acidity or alkalinity. The pH scale ranges from 0 to 14.
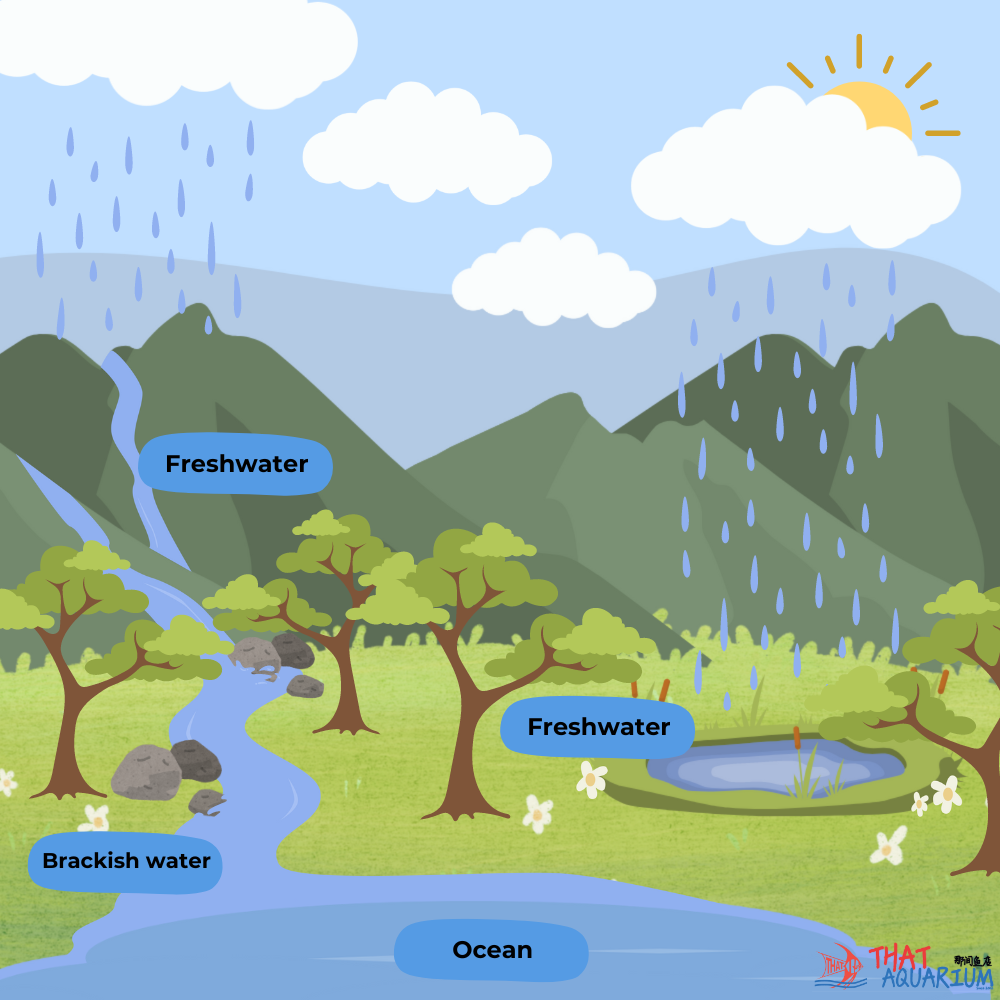
Secondly, as clouds get heavier from continuous condensation of water vapour the resulting water precipitates as rainfall. Depending on where rainwater falls the topography of the ground below will once again after its chemical composition. When water flows over mountainous terrain, especially karst topography its innate carbonic acid will dissolve the overlying calcareous surface; the water, now enriched with calcium carbonate, takes on alkaline pH 7.2-8.5. Examples of freshwater habitats with alkaline water are : Lake Malawi and Lake Tanganyika in East Africa (home to beautiful, dazzling Rift Valley Cichlid species that are popularly kept in home aquariums), as well as many rivers in temperate regions (Europe and North America).
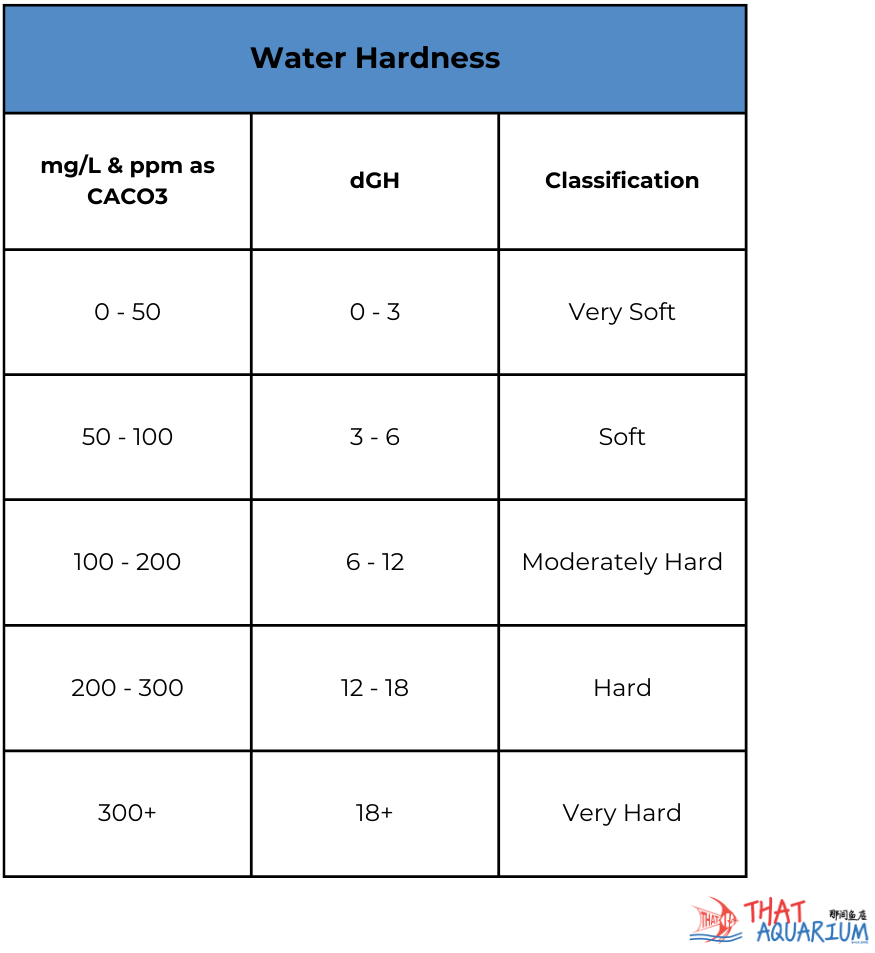
The presence of dissolved carbonates in the water also gives us yet another important measurable water parameter; hardness levels. Before we delve deeper, understand that water hardness is expressed in two terms; GH and KH. What are GH and KH? GH stands for general hardness while KH stands for carbonate hardness. When calcareous materials e.g. limestone dissolves in water, calcium ions, magnesium ions, carbonates and bicarbonates the water is no longer pure H2O and now becomes ‘hard’ due to the four minerals it has now accumulated.
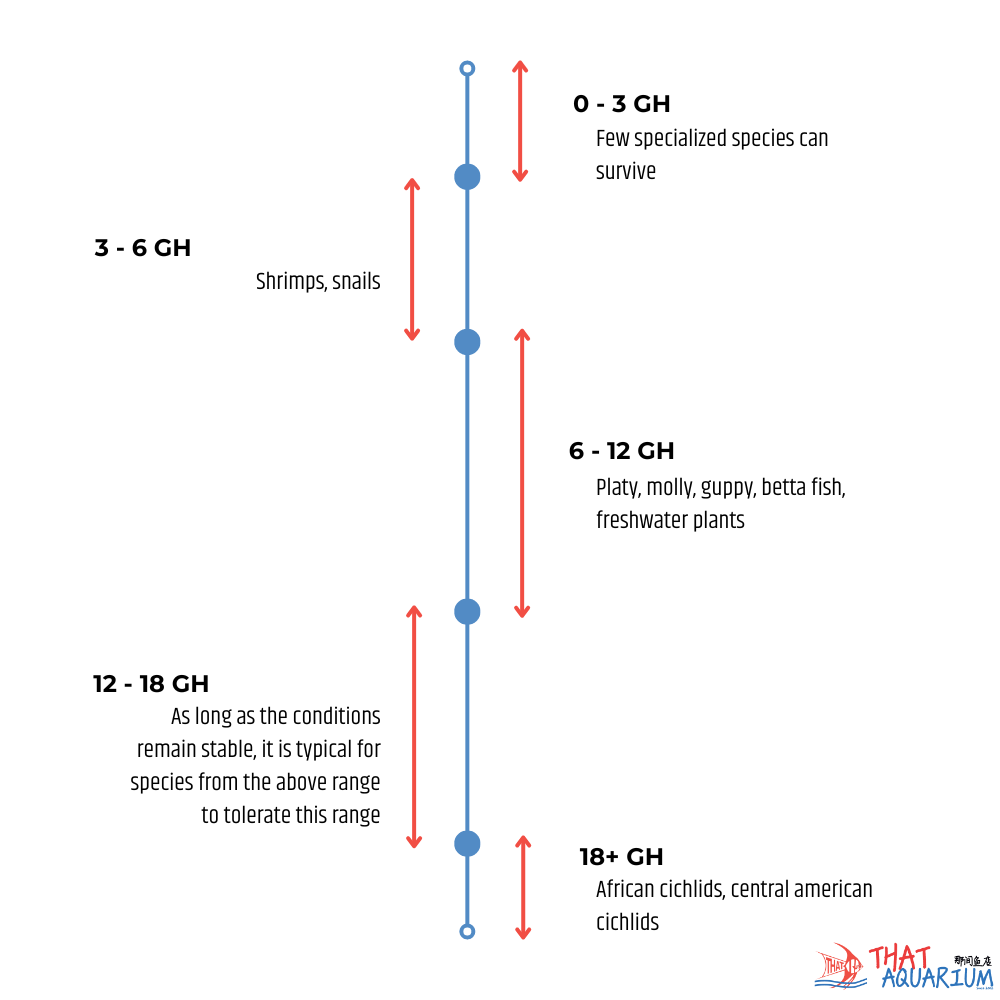
GH is a measure of calcium and magnesium ions while KH is the measure of carbonates and bicarbonates. Confusing? Fret not! Things will only get easier to understand from here. When hobbyists say “My water is hard” they are implying that the GH levels are high. Conversely if the water here is “soft” it means that the GH levels are low. As simple as that! In reality, there are 4 measurable levels of GH (see diagram 2). Namely soft, moderate, hard and very hard. Why do GH levels matter? In Nature, fishes have evolved to adapt to not only specific pH ranges but also a specific GH range too. For example, if we were to rear a sensitive acidophilic (acid water loving) fish such as the liquorice gourami (Parosphromenus spp.) that hail from soft, acidic peat swamps in ordinary tap water with even moderated GH levels the fish will succumb to health issues that will quickly lead to its untimely demise. Thus, the importance of understanding GH levels.
KH is concerned with the water’s buffering capacity aka its ability to help maintain stable pH levels. Are you beginning to see how KH and pH are intertwined, now? That’s great! As mentioned earlier, KH measures the concentration of carbonates and bicarbonates in the water. These 2 compounds are important since they act as buffers to prevent the pH levels from crashing. How? In the aquarium, a natural decomposition process occurs (especially in the filtration unit) that results in the production of acids such as nitric acid. With time, the accumulation of acids will cause the pH to fall; sometimes to dangerously low levels that can negatively impact the health of fishes dwelling in it. Thankfully , Nature has devised an ingenious coping mechanism to counteract said acidification process via buffers.

Dissolved carbonates and bicarbonates help to neutralise acids from reducing the water pH rapidly; thereby helping to resist pH crashing i.e. they provide a “buffering” effect. And it is the measure of this buffering potential which we call KH. Thus, the higher your KH levels the more stable your aquarium’s pH level is going to be. Likewise, the lower your KH levels, the faster pH range and the typically (controlled) crowded housing conditions can quickly acidify the water to dangerously low levels. Atlas, this is why KH is a very important aspect of aquarium water management, in tandem with Nature’s design.
Congratulations on making it this far! We have delved into the topic of water and along the way, the topic of filtration in the aquarium has also been briefly touched upon. Now, we will explore a little further on filtration, as Nature intended for us to emulate in our own aquarium.
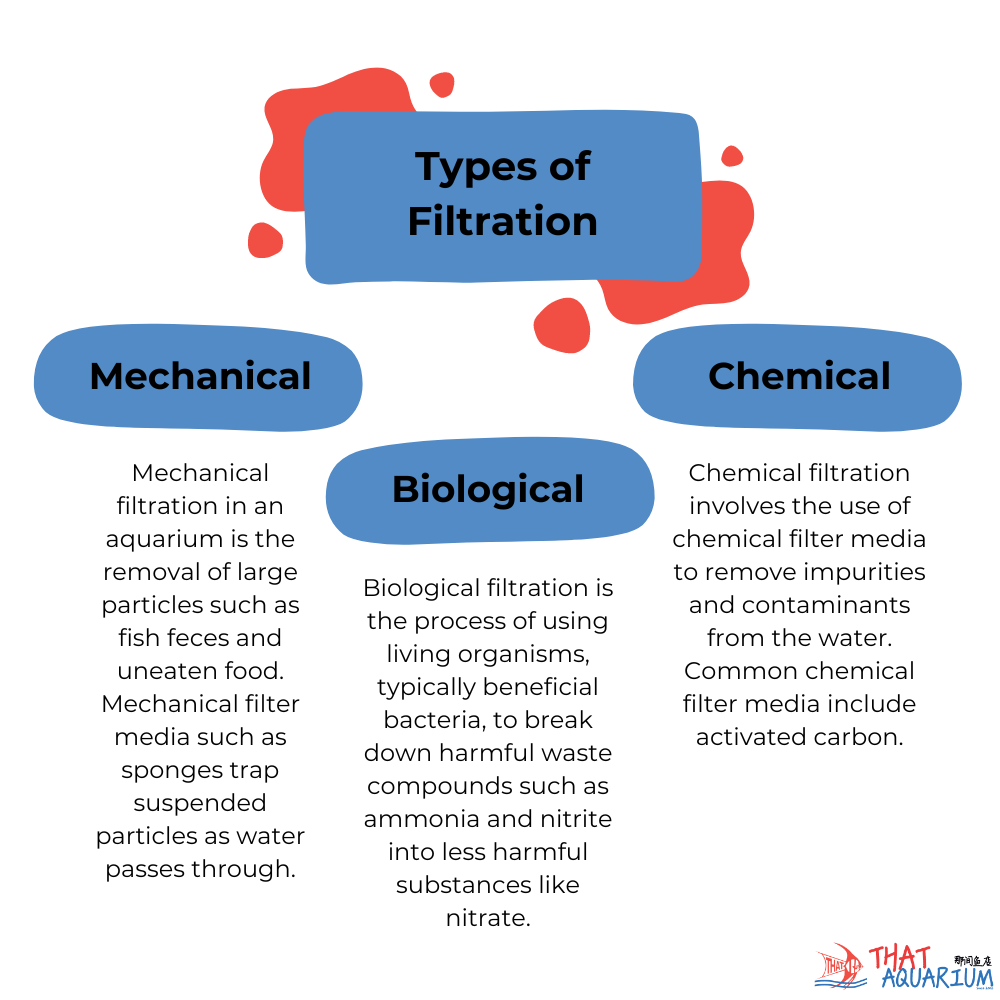
Now, comes the question of “how to dispose of the coffee grounds, then? “Nature provides us with the solution to that problem. All organic matter will eventually decompose. Various decomposers will aid in breaking down waste matter such as bacteria, fungi and archaea. These microscopic organisms are present in our aquariums too! They are Nature’s decomposers and thus, they will eventually find their way into our aquarium naturally (with time) or they can also be introduced immediately, either via an off-the-shelf inoculant product or, courtesy of an established filter. This is the second but most important of filtration called ‘biological filtration‘.
Proudly presented by our That Aquarium Digital Team, where innovation meets aquatic excellence.
Published on 13 June 2025
Author: Saufi
Illustrator(s): Jolin Lee, Nor Haliza, Chai Minyu, Kelvin Phua, Abbie Tan
